India has become a global hub for the manufacturing and supply of bile acid impurities, supporting industries in pharmaceutical research, drug development, and academic studies. Among the leading suppliers, KarpsChem stands out for its expertise in custom synthesis, impurity profiling, and high-purity reference standards. Our inventory includes a wide range of bile acid impurities with defined CAS numbers, essential for analytical method validation, stability testing, and regulatory submissions.
At KarpsChem, all impurities are produced under stringent quality control and global regulatory compliance, ensuring reliability for EP, USP, and BP standards. These impurities are widely cited in research papers, structural studies, and pharmaceutical synthesis projects, making them indispensable for laboratories and companies worldwide.
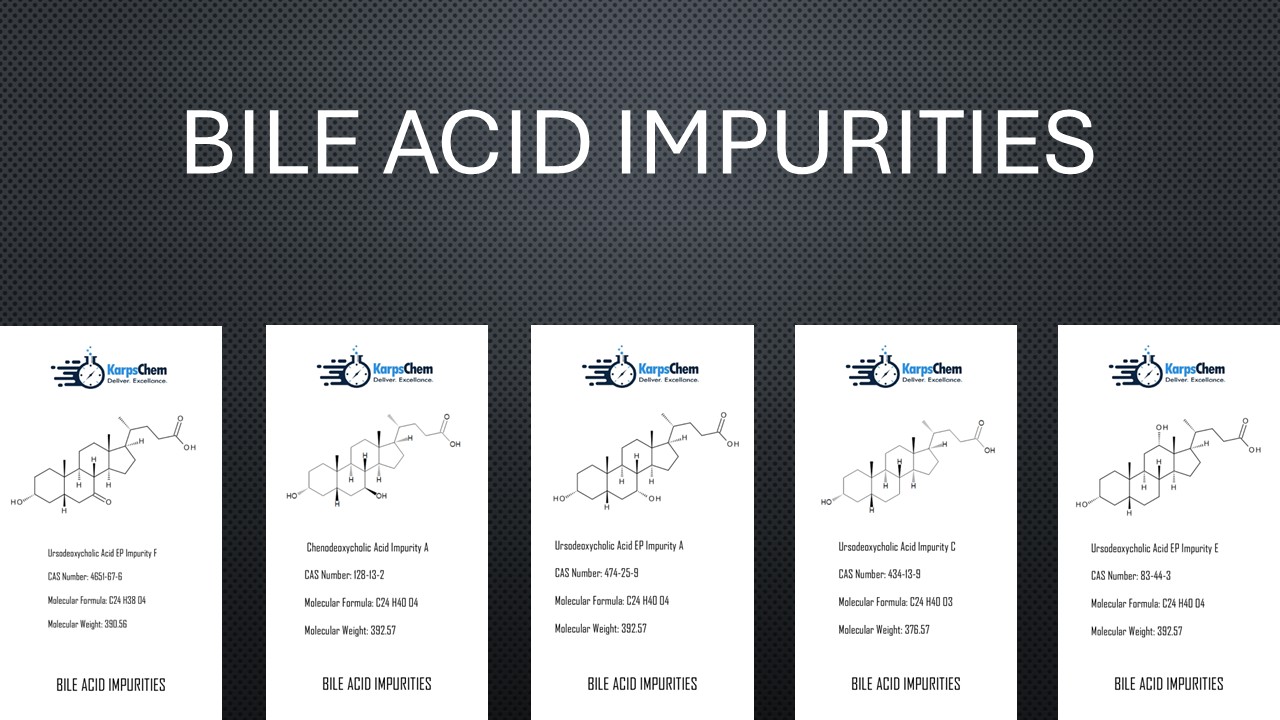
Chenodeoxycholic Acid – Impurity C (CAS 434-13-9)
Also known as Chenodeoxycholic Acid – Impurity C, this compound is a vital reference standard in impurity profiling and analytical method development. It is widely used in pharmaceutical R&D, stability studies, and research papers, providing accuracy in drug substance characterization.
Chenodeoxycholic Acid (CAS 474-25-9) – Ursodeoxycholic Acid EP Impurity A
Recognized as Ursodeoxycholic Acid EP Impurity A, this impurity is important for drug development, impurity analysis, and synthesis studies. It supports analytical validation and pharmacopoeial compliance, ensuring high precision in pharmaceutical quality control.
Chenodeoxycholic Acid EP Impurity E (CAS 83-44-3) – Ursodeoxycholic Acid EP Impurity E
This impurity, known as Ursodeoxycholic Acid EP Impurity E, is frequently used in impurity characterization, synthesis, and research-driven projects. With strong application in regulatory submissions and advanced pharmaceutical analysis, it is one of the most referenced bile acid impurities in scientific publications.
Chenodeoxycholic Acid EP Impurity F (CAS 4651-67-6)
Also referred to as Ursodeoxycholic Acid EP Impurity F, 7-Oxo Ursodeoxycholic Acid, 7-Oxo Chenodeoxycholic Acid, or Nutriacholic Acid (3α-hydroxy-7-oxo-5β-cholan-24-oic acid as per EP), this impurity plays a significant role in drug development, stability studies, and impurity profiling. It is essential for method validation, structural characterization, and regulatory compliance.
Chenodeoxycholic Acid EP Impurity A (CAS 128-13-2)
Known as Ursodeoxycholic Acid (EP), Chenodeoxycholic Acid 7-Epimer, or 3α-7β-dihydroxy-5β-cholan-24-oic acid (as per EP), this impurity is highly valuable in impurity synthesis, research papers, and pharmaceutical testing. It is a key reference material for academic institutions and pharmaceutical companies involved in drug substance analysis.
Why Choose KarpsChem for Bile Acid Impurities?
-
All impurities available in stock for immediate dispatch
-
High-purity, research-grade standards
-
Complete documentation support for regulatory needs
-
Expertise in custom synthesis and impurity profiling
-
Trusted by global pharmaceutical companies, biotech firms, and research organizations
At KarpsChem, we ensure cost-effective, reliable, and globally compliant impurity standards, making us a preferred partner for pharmaceutical R&D, academic research, and industrial applications.


leave a comment