Clotrimazole is a widely used antifungal agent, belonging to the imidazole class, primarily indicated for the treatment of dermatomycoses, candidiasis, and other fungal infections. To ensure regulatory compliance and drug safety, it is essential to identify, synthesize, and study its pharmacopoeial impurities as per EP/BP/USP/ICH guidelines.
At KarpsChem Laboratories Pvt. Ltd., we specialize in providing high-purity pharmaceutical impurities, including the complete range of Clotrimazole EP Impurities A–F. These impurities are critical for drug development, stability testing, toxicological evaluation, impurity profiling, and regulatory submissions across the globe.
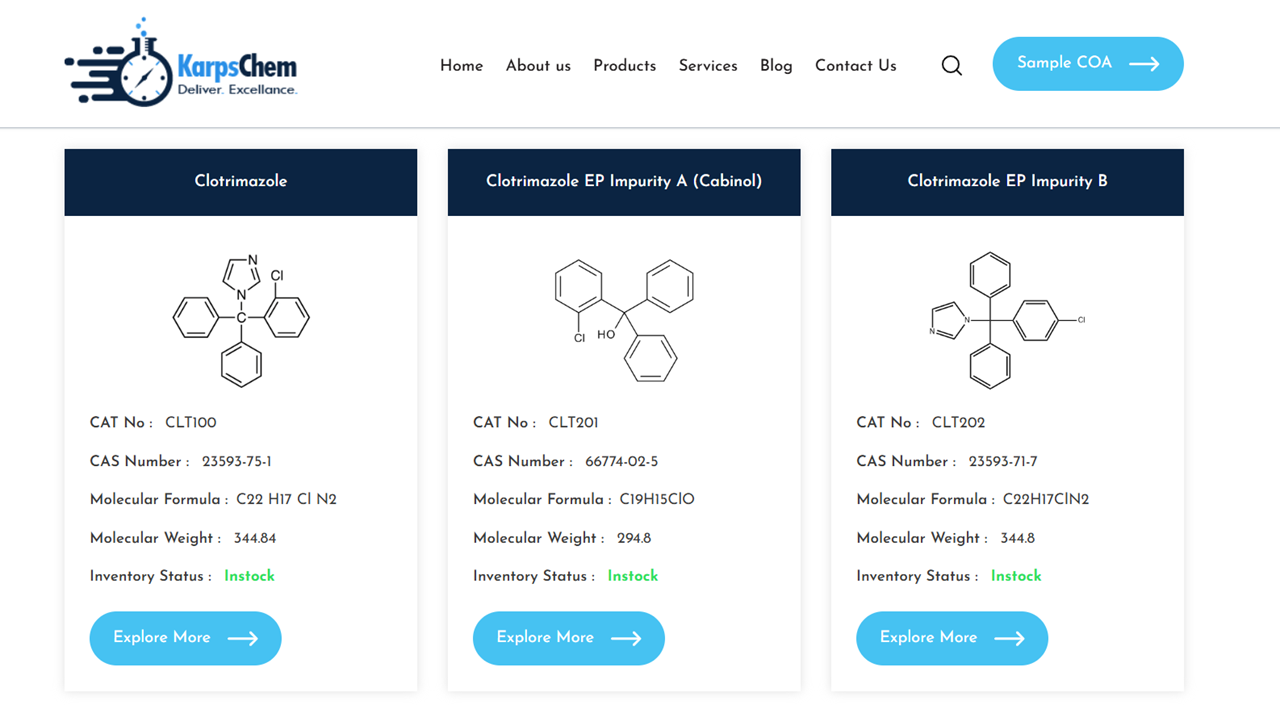
🔬 Clotrimazole EP Impurities Offered by KarpsChem
1. Clotrimazole EP Impurity A (Cabinol) – CAS: 66774-02-5
A by-product of clotrimazole synthesis, Impurity A (Cabinol) is important in process development studies. Its presence must be monitored in accordance with pharmacopoeial monographs.
2. Clotrimazole EP Impurity B – CAS: 23593-71-7
Impurity B is a structural analogue of clotrimazole often formed during synthetic pathways and degradation studies. It is vital for HPLC/LC-MS method validation.
3. Clotrimazole EP Impurity C – CAS: 42074-68-0
Impurity C plays a key role in regulatory toxicology assessments and is typically included in stability-indicating method validation studies.
4. Clotrimazole EP Impurity D – CAS: 288-32-4
An imidazole-related derivative, Impurity D is used in process impurity profiling and is often required in ICH-compliant impurity studies.
5. Clotrimazole EP Impurity E – CAS: 5162-03-8
This impurity is closely monitored in formulation stability and is critical for regulatory submissions in EP/BP/USP pharmacopoeias.
6. Clotrimazole EP Impurity F – CAS: 15469-97-3
A key degradant and synthesis-related impurity, Impurity F is indispensable for toxicology research, impurity quantification, and stability testing.
🌍 Why KarpsChem for Clotrimazole EP Impurities?
-
Global Supplier: Recognized as one of the top impurity manufacturers in India and worldwide.
-
Research-Driven: Supported by peer-reviewed papers, synthesis expertise, and analytical validation data.
-
Regulatory Ready: All impurities come with COA, HPLC, NMR, MS data, aligned with EP/BP/USP/ICH guidelines.
-
Custom Synthesis Expertise: Ability to develop rare and non-pharmacopoeial impurities on demand.
-
Trusted by Pharma Leaders: Supporting top multinational pharma companies, CROs, and academic institutions.
By offering ready stock availability and rapid global delivery, KarpsChem empowers researchers and pharmaceutical industries to accelerate drug discovery, regulatory submissions, and scientific publications.
📌 Applications of Clotrimazole EP Impurities
-
Analytical Method Development & Validation
-
Stability & Degradation Pathway Studies
-
Impurity Profiling & Toxicology Studies
-
Regulatory Filings with EP, BP, USP
-
Research Publications & Academia
📞 Contact KarpsChem Today
🌐 Website: www.karpschem.in
📩 Email: amitnagare@karpschem.in
📱 Call/WhatsApp: +91-9371639228


leave a comment