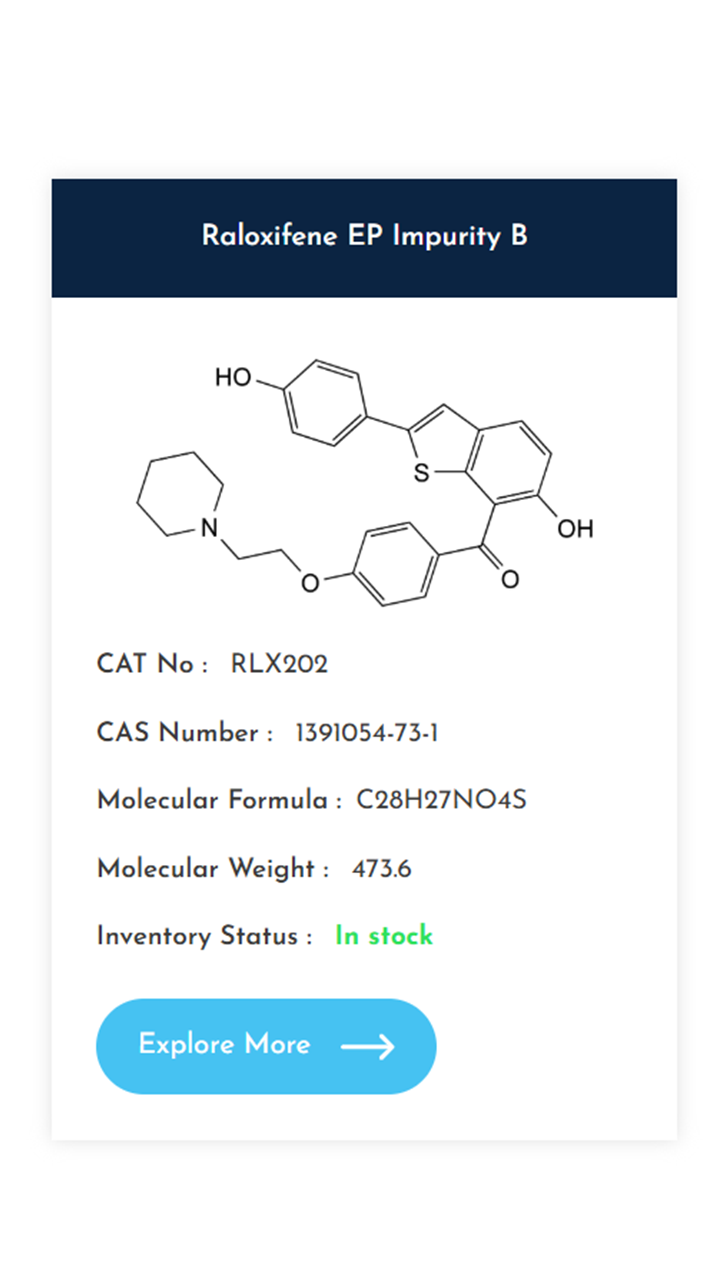
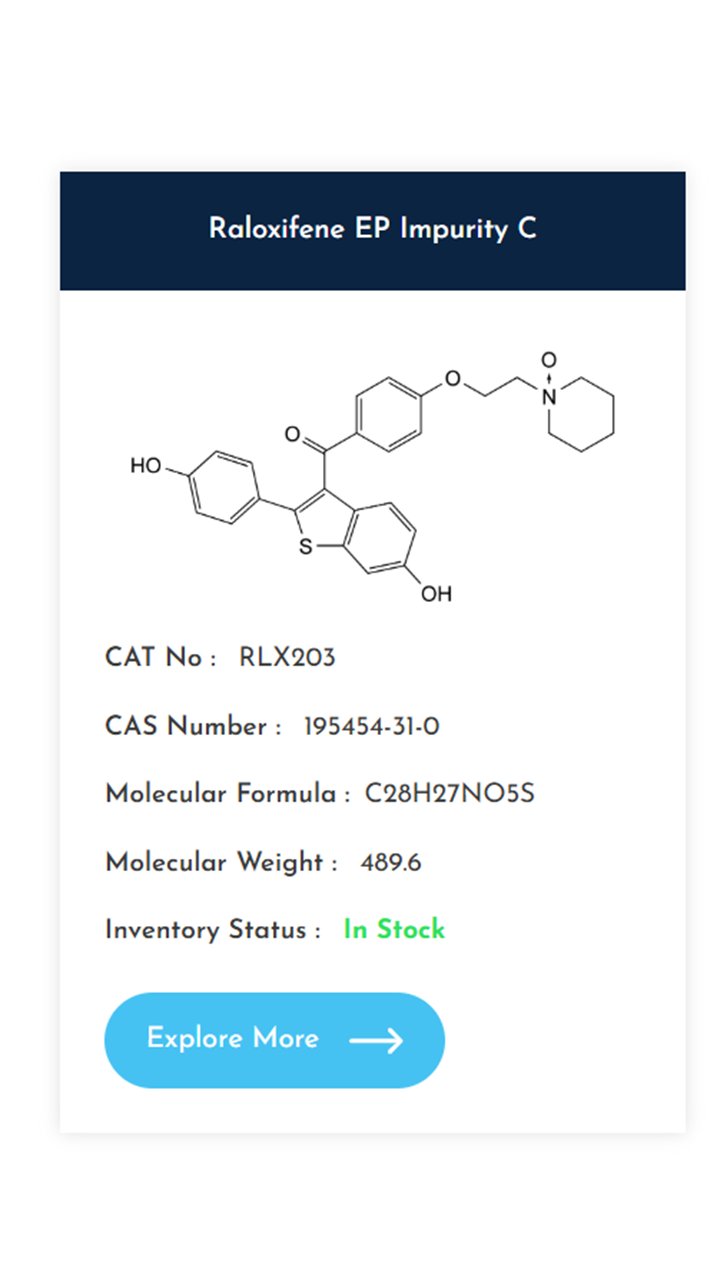
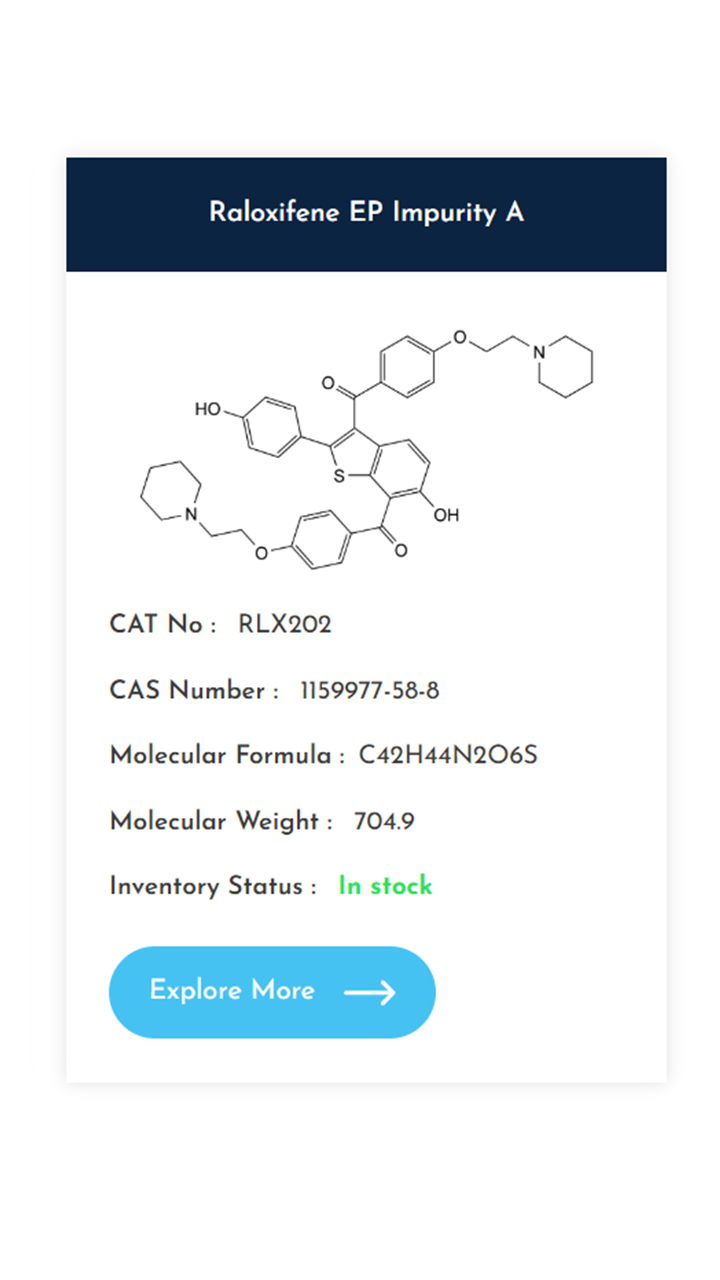
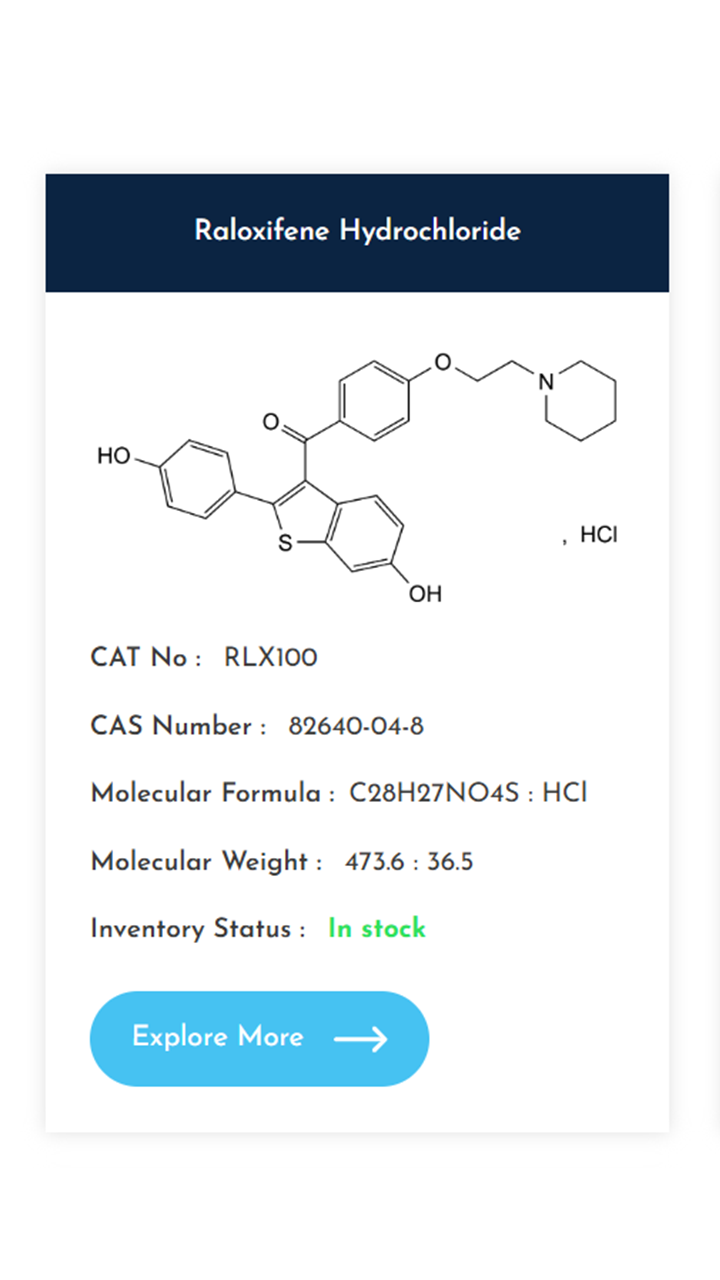
Understanding and controlling impurities is a fundamental requirement in pharmaceutical development. As regulatory expectations continue to rise, access to well-characterized, high-purity impurity standards becomes essential for accurate method validation, impurity profiling, and stability studies. At KarpsChem Laboratories, we are committed to supporting the pharmaceutical industry with reliable, research-grade impurity standards synthesized under strict quality systems. […]


All Glycol Impurity Derivatives Now In-Stock at KarpsChem Laboratories
At KarpsChem Laboratories, we continue to expand our high-purity impurity standards portfolio to support global pharmaceutical, chemical, and research companies. We are pleased to announce that a comprehensive range of Glycol impurities and their derivatives are now readily available in stock. Available Glycol Impurity Standards: Ethylene Glycol Monoisopropyl Ether Polyethylene Glycol 6000 (PEG 6000) Diethyl […]
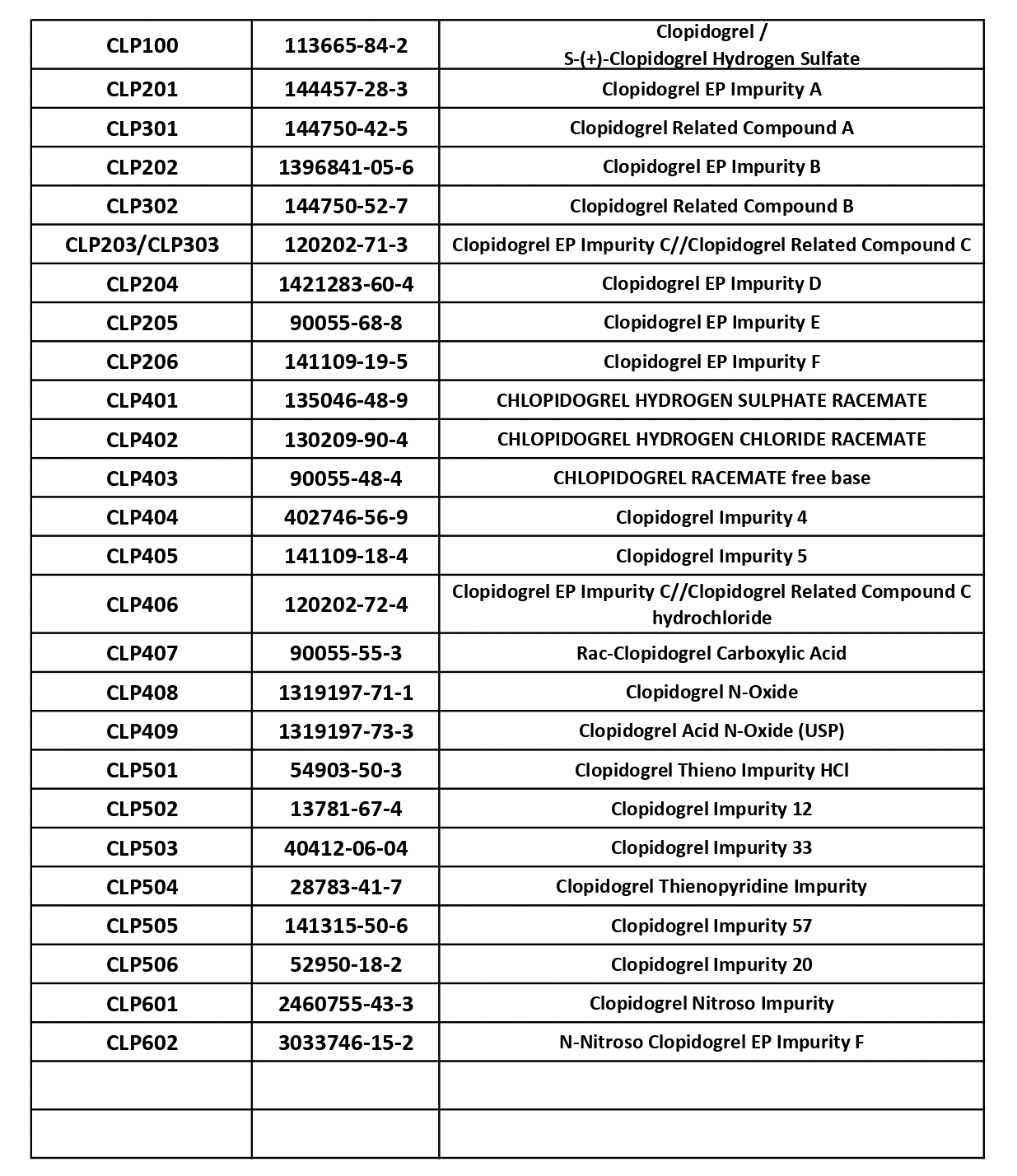
Clopidogrel Impurities – Complete Solutions Available at KarpsChem
Clopidogrel is one of the most widely used antiplatelet drugs in cardiovascular therapy, and accuracy in impurity profiling plays a critical role in ensuring its quality, safety, and regulatory compliance. As pharmaceutical companies continue to advance research and generic drug development, the demand for high-purity Clopidogrel impurities and reference standards has increased dramatically. At KarpsChem, […]
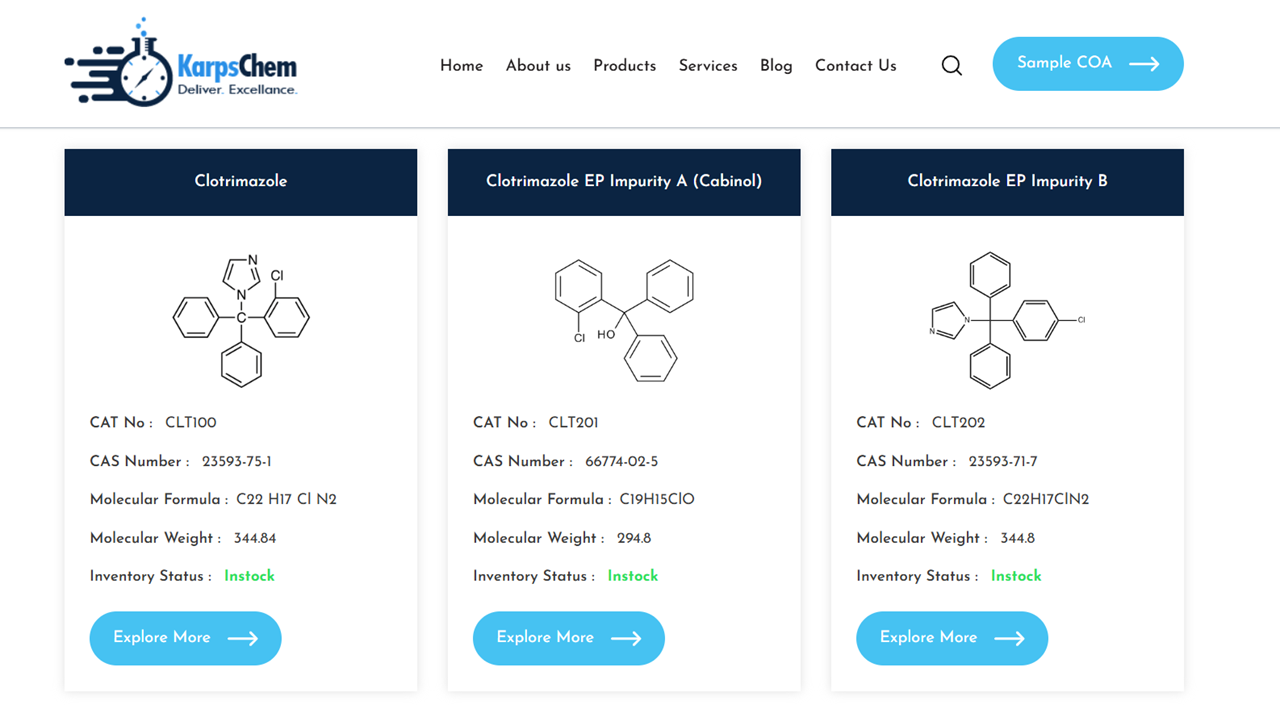
Comprehensive Guide to Clotrimazole EP Impurities (A–F) | High-Purity Standards at KarpsChem
Clotrimazole is a widely used antifungal agent, belonging to the imidazole class, primarily indicated for the treatment of dermatomycoses, candidiasis, and other fungal infections. To ensure regulatory compliance and drug safety, it is essential to identify, synthesize, and study its pharmacopoeial impurities as per EP/BP/USP/ICH guidelines. At KarpsChem Laboratories Pvt. Ltd., we specialize in providing […]
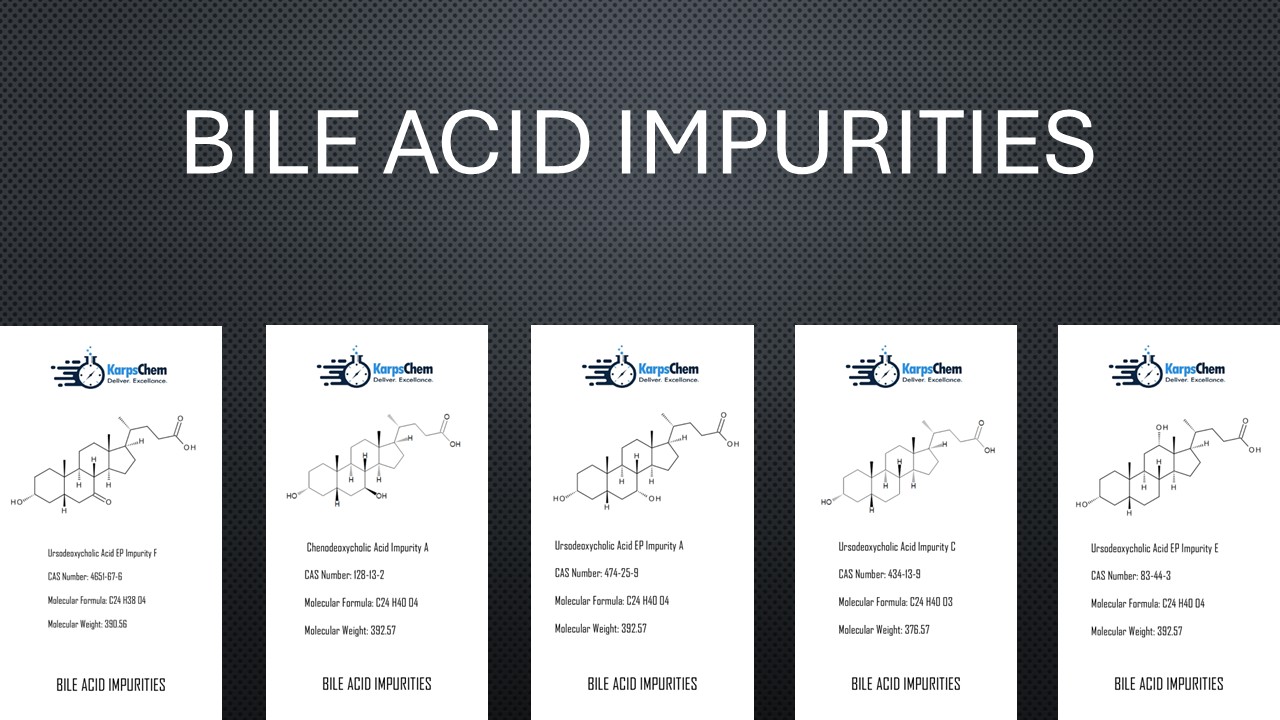
Bile Acid Impurities – High-Purity Reference Standards Available at KarpsChem
India has become a global hub for the manufacturing and supply of bile acid impurities, supporting industries in pharmaceutical research, drug development, and academic studies. Among the leading suppliers, KarpsChem stands out for its expertise in custom synthesis, impurity profiling, and high-purity reference standards. Our inventory includes a wide range of bile acid impurities with […]

🧪 N-Nitroso Impurities Now In Stock at KarpsChem – Stay Compliant, Stay Ahead!
🚨 Concerned about Nitrosamine Contamination?You’re not alone. Regulatory bodies across the globe are cracking down on N-Nitroso impurities, demanding trace-level detection and impurity profiling across APIs and formulations. Failure to comply can lead to costly recalls and regulatory holds. At KarpsChem Laboratories Pvt. Ltd., we’ve got your back with high-purity N-Nitroso impurities—synthesized in-house, verified by […]

Buy Memantine HCl & Related Impurities From KarpsChem Laboratories Pvt. Ltd.
Are you looking for high-purity Memantine Hydrochloride impurities for analytical R&D or formulation development?KarpsChem Laboratories Pvt. Ltd., a reputed manufacturer and global supplier of pharmaceutical impurities and intermediates, now offers Memantine HCl and all its related compounds in stock, available for immediate supply with detailed analytical data and regulatory support. 🧠 What is Memantine HCl? […]

Suvorexant Impurities & Reference Standards – In Stock at KarpsChem
Suvorexant is a dual orexin receptor antagonist (DORA) approved for the treatment of insomnia. During the development and quality control of Suvorexant drug substances and drug products, it is essential to identify, quantify, and control process-related impurities, degradation products, and isomeric forms to meet regulatory guidelines set by authorities such as the FDA, EMA, and […]

Hurry! Etoricoxib EP Impurities Are Now Instock in Karpchem Laboratories Pvt. Ltd
We are thrilled to announce that Etoricoxib EP Impurities are now in stock at Karpchem Laboratories Pvt. Ltd.! These impurities are designed to meet the stringent standards of the European Pharmacopoeia (EP), ensuring reliable and precise applications for your pharmaceutical and research needs. Why Choose Karpchem Laboratories Pvt. Ltd? High-Quality Standards: Our Etoricoxib EP Impurities […]