Clopidogrel is one of the most widely used antiplatelet drugs in cardiovascular therapy, and accuracy in impurity profiling plays a critical role in ensuring its quality, safety, and regulatory compliance. As pharmaceutical companies continue to advance research and generic drug development, the demand for high-purity Clopidogrel impurities and reference standards has increased dramatically.
At KarpsChem, we support global pharmaceutical R&D and QC laboratories by supplying a complete portfolio of Clopidogrel impurities, including pharmacopeia and non-pharmacopeia standards. Each impurity is synthesized with high precision, characterized through advanced analytical techniques, and provided with full documentation to support regulatory submissions.
Why Clopidogrel Impurity Standards Matter
Clopidogrel impurities arise from:
-
Synthetic process by-products
-
API degradation
-
Intermediates or related substances
Accurate analysis of these impurities is essential to:
-
Maintain API purity
-
Ensure patient safety
-
Comply with ICH and regulatory requirements
-
Support method development and validation
KarpsChem offers reference-grade and working-standard materials to help laboratories meet regulatory expectations with confidence.
Complete List of Clopidogrel Impurities Available at KarpsChem
The following Clopidogrel impurities are available at KarpsChem:
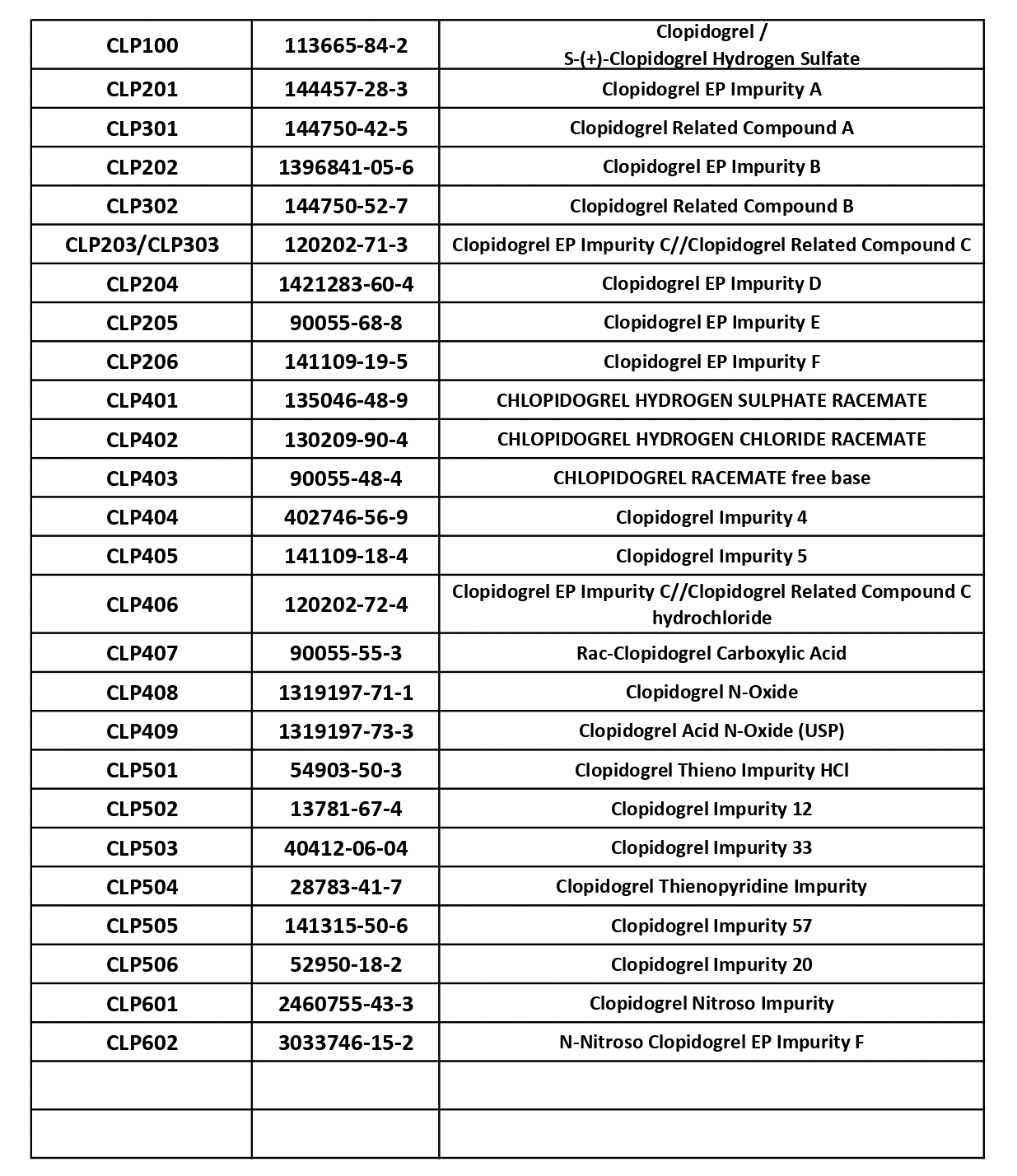
S-(+)-Clopidogrel Hydrogen Sulfate (CLP100, CAS 113665-84-2), Clopidogrel EP Impurity A (CLP201, CAS 144457-28-3), Clopidogrel Related Compound A (CLP301, CAS 144750-42-5), Clopidogrel EP Impurity B (CLP202, CAS 1396841-05-6), Clopidogrel Related Compound B (CLP302, CAS 144750-52-7), Clopidogrel EP Impurity C / Clopidogrel Related Compound C (CLP203/CLP303, CAS 120202-71-3), Clopidogrel EP Impurity D (CLP204, CAS 1421283-60-4), Clopidogrel EP Impurity E (CLP205, CAS 90055-68-8), Clopidogrel EP Impurity F (CLP206, CAS 141109-19-5), Chlopidogrel Hydrogen Sulphate Racemate (CLP401, CAS 135046-48-9), Chlopidogrel HCl Racemate (CLP402, CAS 130209-90-4), Chlopidogrel Racemate Free Base (CLP403, CAS 90055-48-4), Clopidogrel Impurity 4 (CLP404, CAS 402746-56-9), Clopidogrel Impurity 5 (CLP405, CAS 141109-18-4), Clopidogrel Related Compound C Hydrochloride (CLP406, CAS 120202-72-4), Rac-Clopidogrel Carboxylic Acid (CLP407, CAS 90055-55-3), Clopidogrel N-Oxide (CLP408, CAS 1319197-71-1), Clopidogrel Acid N-Oxide USP (CLP409, CAS 1319197-73-3), Clopidogrel Thieno Impurity HCl (CLP501, CAS 54903-50-3), Clopidogrel Impurity 12 (CLP502, CAS 13781-67-4), Clopidogrel Impurity 33 (CLP503, CAS 40412-06-04), Clopidogrel Thienopyridine Impurity (CLP504, CAS 28783-41-7), Clopidogrel Impurity 57 (CLP505, CAS 141315-50-6), Clopidogrel Impurity 20 (CLP506, CAS 52950-18-2), Clopidogrel Nitroso Impurity (CLP601, CAS 2460755-43-3), and N-Nitroso Clopidogrel EP Impurity F (CLP602, CAS 3033746-15-2).
All materials are supplied with COA, NMR, LC-MS, HPLC purity, and supporting analytical data.
Why Global Researchers Trust KarpsChem
✔ Ready-stock Clopidogrel impurities to avoid research delays
✔ High-purity analytical standards with reproducible results
✔ Competitive pricing to reduce R&D cost
✔ Fast worldwide delivery with secure packaging
✔ 24×7 data and technical support from expert scientists
With a specialized team in impurity synthesis and analytical characterization, KarpsChem continues to be a trusted partner to leading pharmaceutical and CRO labs worldwide.
Request Quote or Stock List
To receive a full list of in-stock Clopidogrel impurities and pricing, contact us:
📩 sales.karpschem@gmail.com
📩 amitnagare@karpschem.in
KarpsChem — Delivering Excellence in Pharmaceutical Impurity Standards and CRO Solutions Worldwide.


leave a comment